Discover the Future of Medicine and Longevity
The pandemic of 2020 focused our attention on the importance of efficient medical research and the need to revamp the current government-certification process to move experimental treatments more quickly from lab to patient. But looking beyond immediate COVID-19 challenges should give us more optimism about our future than at any time in human history.
This is because we will see that it’s no longer science fiction to assert that individuals, perhaps in mere decades to come, will be able to live healthy lives for 100 years, or even 200 and longer.
Exponential technology is allowing us not only to understand and devise treatments for diseases and ailments that have plagued humanity through its history, but for the aging process itself.
The research is clear: aging no longer should be thought of as an inevitability that will eventually kill us all. Rather, it should be understood as a disease that can be treated and even cured.
The path to this future, however, will see many of other diseases and ailments swept away first, but only if that path is cleared of government barriers blocking innovators and mental barriers blocking our understanding that, yes, such a future is possible.

Consider the context. The communications and information revolution—with the creation of personal computers, smartphones and other tech—already has transformed the economy, society and culture. This revolution and associated technologies are the basis of the next revolution that is leading us to longer, healthier lives.
- Robots and robotic systems, for example, assist with hospital service hospitals, from meal deliveries, to administering tests, to even performing surgeries, allowing physicians with special goggles and video access to operate remotely.
- Smartphones and watches already offer us wearable diagnostics, track exercise activities, heart rates, hydration levels, calories burned, and much more. An Apple Watch and now iPhones have an ECG that can monitor the heart. Lab-on-a-chip devices can analyze a tiny among of fluid of a patient for a number of conditions. We’ll soon see widespread use of wearable diagnostics. Imagine a shirt embedded with various sensors that can perform full checkups. The Oura ring currently helps track bio-rhythms in persons with sleep disorders. Imagine a ring or watch in the future that detects when a patient is in imminent danger of a heart attack and alerts medical personnel to speed to their door for the emergency occurs.
- Artificial Intelligence and machine learning will have a central role to play in future healthcare. Already, the IBM Watson supercomputer, for example, has reviewed thousands of MRIs and now can make medical evaluations as good as teams of physicians and even new medical research discoveries.
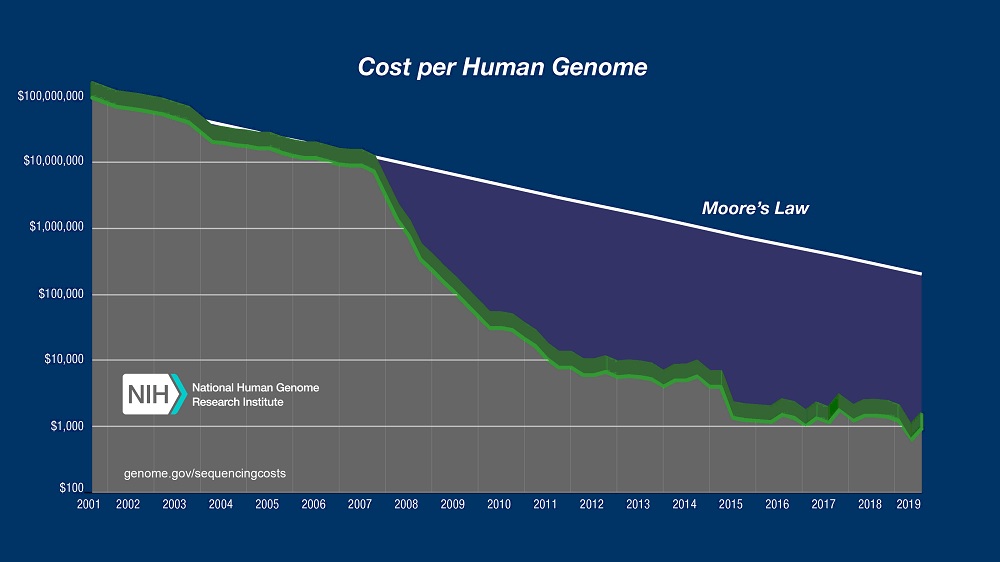
- The preliminary mapping of the human genome, the underlying code in DNA that generates we human beings, was done in 2001. The actual cost of sequencing a human-sized genome at that time was estimated at around $100 million. (The projects that led to this mapping cost much more.) By 2007 the cost was around $10 million. Today, it is only about $1,000. Companies now can determine exactly what medical problems you’re prone to so you can take preventive measures. Big data, with AI systems reviewing many such scans will certainly lead to even more breakthroughs.
- •Gene editing tools like CRISPR-Cas9 now make it possible to actually genetic materials that dispose one to particular diseases. Imagine a world in which such tools can head off Alzheimer’s or Parkinsons!
- In April 2018, a breakthrough that should have been heralded around the world was made. The enzyme telemerase was sequenced. This is important because each chromosome has little lengths of materials called “telomeres” on its end. As chromosomes reproduce over time, the telomeres shorten and when they’re no more, reproduction stops. We age and die. Researchers now can work with telemerase to keep telomeres intact, meaning they could keep us as individuals intact—alive and healthy!
This is the bright future that exponential technology is ushering in.
We all, of course, want any new treatments to be both safe and effective. But we also need them delivered in a timely manner. Here where a souring healthcare future is chained to the past.
The U.S. Food and Drug Administration (FDA) in its current iteration was created in 1962 to certify the safety and efficacy of proposed new treatments and medical devices. The system of double-blind trials with control groups receiving placeboes and other receiving the actual experimental product is rigorous but extremely costly and time-consuming. A 2014 Tufts study found it takes 10-12 years at a cost of almost $3 billion to bring a new product from research lab to patient, with the certification process accounting for much that time and costs. Thousands of people suffer and die in the interim, and many researchers in small labs simply can’t afford those costs, so innovative efforts are stillborn.
Indeed, a 1981 a Wall Street Journal headline declared, “100,000 killed” because of the FDA’s eight-year delay approving beta blockers to regulate hypertension and heart problems, even as the product was available in other developed countries.
The FDA has made ad hoc reforms to over the years to speed the approval process. But it’s still too slow and will prove even more antiquated when dealing with breakthroughs with exponential technologies. For example, in April 2019, the FDA sought input on a “Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD).” Of course, AI software is not a medical device, but FDA must fit this square AI peg in its regulatory round hole.
The more exponential medical technology advances in the future, the more the FDA’s antiquated certification system will get in the way pf progress.
The FDA needs a top-to-bottom review is it to help deliver safe, effective treatments quicker and at lower costs. Reforming huge bureaucracies is difficult.
Currently, a proposed new treatment, after several years of preclinical development in research labs, enters Phase I testing for basic safety. Safe products then enter years of multiple Phase II tests to measure efficacy. Phase III testing is then required to refine efficacy and proper dosage. Only then can a product receive a final review and, after perhaps a decade, be certified.
An important and obvious reform would be the Free To Choose Medicine (FTCM), pioneered by entrepreneur Bartley Madden. Once a promising new drug passes Phase I safety trials and at least one Phase II efficacy trial, a manufacturer can opt to offer the product on a parallel FTCM track. Patients, in consultation with their physicians, could either access these promising FTCM medications or continue to wait for drugs still languishing for years in the current FDA process.
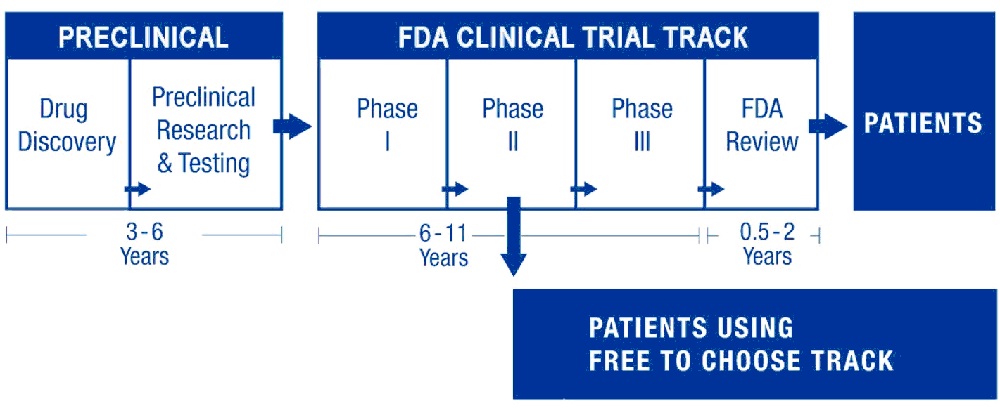
A predecessor parallel track to the FTCM system was created in in 1992 that allowed some 12,000 AIDS suffers to access a life-saving medication still in FDA trials. The Japanese government has created a similar track for regenerative medicine products.
This parallel track option would dovetail well with the growing revolution in medical treatments. The experience of patients using a product on the FTCM track would have results, with privacy protected, recorded in a tradeoff evaluation drug database that could be accessed by patients and researchers alike. The advantages of studying the effects of a product using a steam of real-world versus in controlled patient-group tests would be similar to those of studying a tiger in the wild versus in a zoo cage. The former offers a better overall understanding. Such an approach to collecting and utilizing medical data would allow less-promising proposed treatments to “fail faster” and the most promising to stand out quicker and receive more prompt certification.
This and other reforms can be helped along if researchers themselves ask, “What kind of system would best facilitate my own efforts to develop breakthrough treatments for various ailments and to extend life, a system that would ensure patient safety but be appropriate for exponential technologies?”
The future for our health and longevity is could not be brighter. But citizens, researchers, and policymakers alike need to appreciate that such a future is, indeed, no longer science fiction. They need to understand what is really possible in the future and what sort of innovative thinking is needed to usher in that future. They need to understand the need for young people especially to be inspired by the vision of tomorrow that sees disease after disease banished from the human scene so they will commit their lives to the work needed to create that tomorrow because it will mean their own lives and go one and on from achievement after achievement and joy after joy.
Let make the creation of that tomorrow our highest priority!
Future Medicine and Life Extension Items.
- September 17, 2024, Blind Spots: When Medicine Gets It Wrong, and What It Means for Our Health, by Marty Makary.
- June 11, 2024, "Antitrust Against Apple and Its AI Could Endanger Your Life," by Edward Hudgins.
- April 9, 2024, ChatGPT, MD: How AI-Empowered Patients & Doctors Can Take Back Control of American Medicine, by Robert Pearl, MD.
- September 29, 2023, "International Longevity Day and a Death-Defying Congressional Caucus," by Edward Hudgins.
- October 4, 2022, "From 'Sickcare' to Healthcare and Your 200th Birthday!" by Edward Hudgins.
- August 24, 2021, The Science and Technology of Growing Young, by Serge Young.
- December 29, 2020, Progress, Potential, and Possibilities video interview with Bartley Madden on "Free To Choose Medicine: Better Drugs, Sooner, at Lower Costs, Saving Lives."
- September 4, 2020, “Seniors suffer the most from antiquated FDA approval process, by Diane Abbitt and Bartley Madden.
- September 2, 2020, "A Revolution Against Aging and Death Is Not Scifi," by Edward Hudgins
- March 2020, Science on FDA Liberalization: A Response to the Status Quo Process for Medical Treatments, by Bartley Madden.
- September 10, 2019, The Price We Pay: What Broke American Health Care--and How to Fix It , by Marty Makary.
- September 2019, Lifespan: Why We Age--and and Why We Don't Have To, by David A. Sinclair, with Matthew D. LaPlante.
- April 2019, Policy Brief: A Modern System for Approving the Cures of The Future, by Edward Hudgins
- March 1, 2019, Epoch Times/CPAC Video Interview, “Edward Hudgins on The Coming Medical Revolution.”
- February 2019, Policy Brief: How Extending The AIDS Drug Access Model to Other Diseases Would Save Lives, by Edward Hudgins.
- October 10, 2018, “Cure for cancer would become more likely if FDA streamlined the drug approval process,” by Edward Hudgins.
- June 2018, Policy Brief: Free To Choose Medicine in Japan: A Model for America, by Edward Hudgins
- April 2018, Free To Choose Medicine: Better Drugs Sooner at Lower Cost: 3rd Edition, by Bartley Madden.
- July 28, 2016, “Public Opposition to Biotech Endangers Your Life and Health,” by Edward Hudgins
- May 18, 2016, “Which Culture Can Make 120 Years Old the Prime of Life?”, by Edward Hudgins.
- March 8, 2016 Genomics and Personalized Medicine: What Everyone Needs to Know by Michael Snyder.
- December 3, 2015, “Will Banning Genetic Engineering Kill You?” by Edward Hudgins.
- November 20, 2015, “On Viewing 2001: The First Transhumanist Film,” by Edward Hudgins.
- April 22, 2015, “How anti-individualist fallacies prevent us from curing death,” by Edward Hudgins
- March 12, 2015, “Google, Entrepreneurs, and Living 500 Years,” by Edward Hudgins.
- December 11, 2013, “FDA Stopping the Genetics Revolution,” by Edward Hudgins.
- January 1, 2014, Bill Andrews on Telemere Basics: CURING AGING, by Bill Andrews, Ph.D., and Jon Cornell.
- April 23, 2013, 100 Plus: How the Coming Age of Longevity Will Change Everything, From Careers and Relationships to Family and Faith, by Sonia Arrison.
- January 2008, Ending Aging: The Rejuvenation Breakthroughs That Could Reverse Human Aging in Our Lifetime, by Aubrey DeGrey, with Michael Rae.